As of the 1st of January 2025, Bellberry Fees will be updated. The fee incorporates CPI impacts and a the review and ongoing oversight of complex Multi-site clinical trial applications (i.e Tele-health clinical trials).
The fee for review schedule can be found on the Bellberry website.
The distinction between multi-centre and multi-site (also known as a satellite/decentralised site) applications is a common question at Bellberry. We will clarify these terms by providing examples, highlighting their differences, and explaining the associated fee structures.
Whether participating locations under the oversight of a Principal Investigator meet the definition of multi-centre or multi-site are considered by the Bellberry team and reviewing HREC.
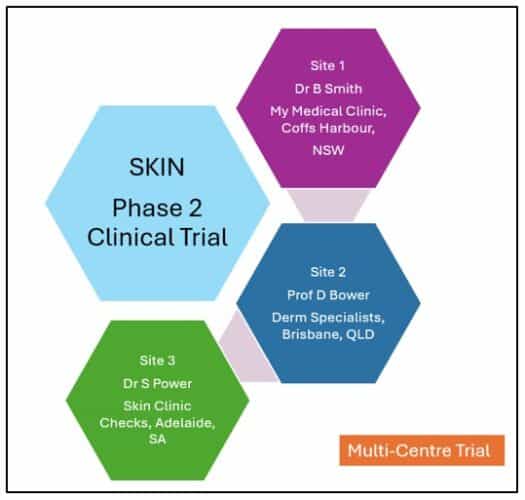
Multi-centre applications refer to research studies conducted at multiple locations, each under the oversight of a different Principal Investigator (PI). Each site conducts the study independently within the study framework, requiring distinct ethical oversight and governance processes at each participating location.
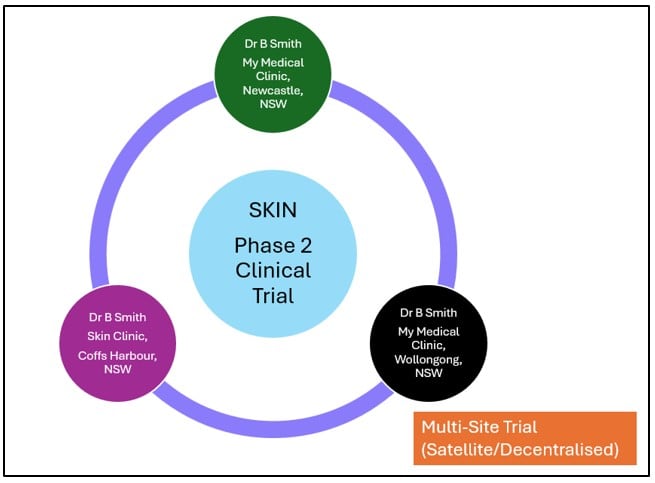
In contrast, multi-site (satellite/decentralised) applications involve research conducted across multiple locations under the oversight of a single Principal Investigator (though the study may be conducted at other multi-centre locations).
A multi-site application typically centralises the study’s management, with the PI taking responsibility for the conduct and oversight of the study at the locations affiliated with that application. For instance, in a tele-trial or decentralised model, a primary site may coordinate elements of the research activities across multiple locations while maintaining unified oversight under the same PI.
Studies can also be multi-centre and multi-site as per the image below:
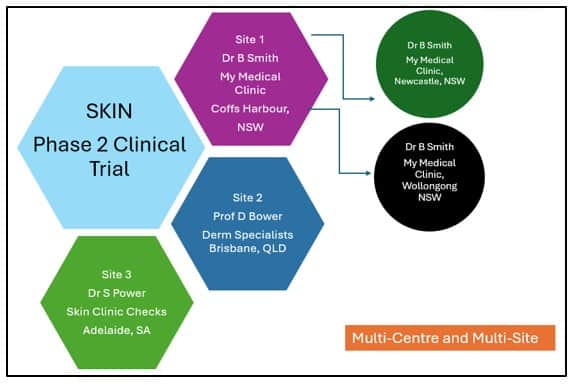
Like multi-centre applications, multi-site applications require administration to ensure robust governance, consistent implementation, and compliance with regulatory and ethical standards across all participating locations. This requires additional resourcing from the HREC and will incur an additional fee from 1 January 2025.
Please note that this will be prospectively introduced and will not impact existing applications, unless an amendment to include a multi-site location is submitted to the HREC for review. Non-clinical trials, such as registries or vendor sites will not incur a fee for multi-site locations.
If you have any queries, please email [email protected]



